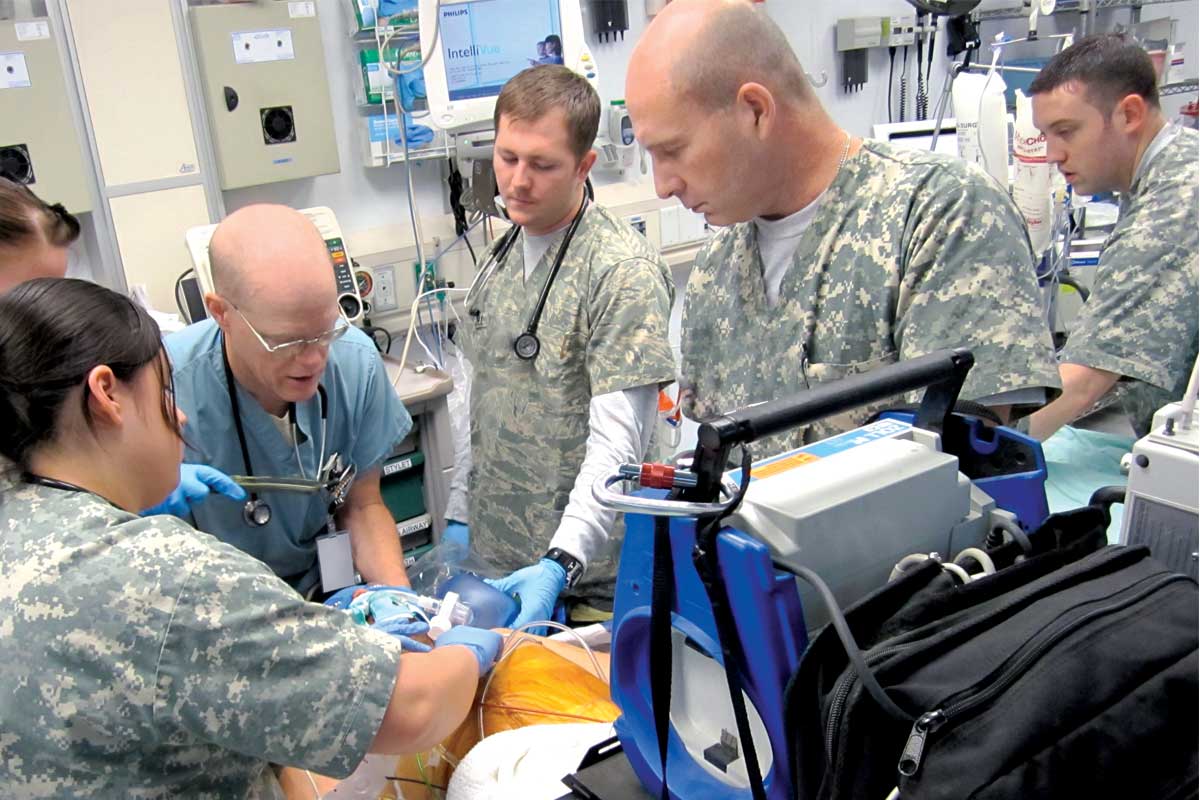
An Air Force trauma team is activated at Bagram Air Base in Afghanistan.
In a war zone in Afghanistan, a roadside bomb explodes and shrapnel tears through a U.S. soldier. The blast hurls him to the ground, and blood begins to flow from multiple wounds.
The soldier described here — a composite based on actual cases — faces a terrifying prospect: losing too much blood to survive before making it to a medical center. Bleeding out is the leading cause of potentially survivable deaths on the battlefield.
In this sandblasted combat setting, military medics must race to slow the bleeding and get the soldier to better-equipped facilities. To do this, they lean on skills they learned more than 7,000 miles away at Washington University School of Medicine prior to deployment. Their efforts are the first step in a long chain of military actions that ultimately will bring the injured war fighter home.
Surgeons and researchers here are training military medics in frontline readiness, inventing ways to control and replace blood loss, and shaping U.S. military-civilian trauma care policies. These efforts are positioning St. Louis as a national hub for research and control of traumatic bleeding, which could save not only lives like the soldier’s, but that of civilians, too.
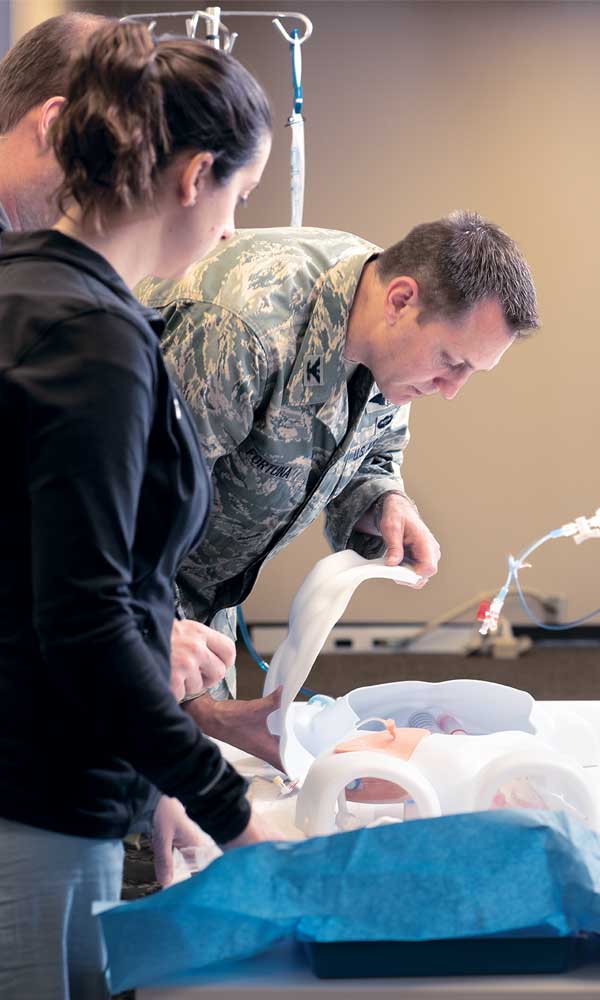
Col. Gerald Fortuna, MD, director of the C-STARS program and a vascular surgeon at the medical school, leads a REBOA training exercise. Developed by the military, REBOA (Resuscitative Endovascular Balloon Occlusion of the Aorta) is a relatively new blood-control technique only available at a handful of medical institutions. Using an ultrasound wand, trauma medics can guide placement of a catheter into the femoral artery and inflate a balloon to block the blood vessel. That way, a patient’s heart can still pump blood to the brain, without pumping it out of the open wound.
When the wounded soldier in Afghanistan is stable enough to move, a helicopter flies him to the next triage station: a tent hospital with equipment stored in canvas bags for easy transport in an emergency.
Doctors invoke a “walking blood bank,” and military personnel pre-screened for blood donation rapidly assemble. Within 30 minutes, one unit of whole blood is collected, and then transfused to the patient, while a surgical team works to control the bleeding.
Pre-deployment trauma training
Before entering the war zone, many of these medical practitioners were trained in situations that mimic battlefield conditions — and they did it in St. Louis. The School of Medicine is the newest partner, along with the U.S. Air Force and SSM Saint Louis University (SLU) Hospital, in a national program called the Center for Sustainment of Trauma and Readiness Skills (C-STARS).
In C-STARS, military medical technicians, nurses and doctors undergo 19 two-week sessions yearly at one of five national training sites. Covered topics include: care under fire, pre-hospital triage, hemorrhage control and the particulars of air evacuation. In short, medics are trained to use the latest field-ready innovations to sustain life.
When these physicians and medics are not deployed to a war zone, they may work in private practice or be reservists on military bases in the U.S. Their daily work typically is nothing like a war hospital. Yet, next year or the year after, they may get redeployed and thrown into situations where they need to be at the top of their game.
“It’s easy for skills to erode if you spend too much time at your home base,” said Col. Gerald Fortuna, MD, director of the St. Louis C-STARS program and a vascular surgeon newly recruited to the School of Medicine. He holds joint appointments at Washington University and Saint Louis University.
“Back at home, the No. 1 surgical procedure in our hospitals is delivering babies,” he said. “That’s drastically different than being over in Iraq or Afghanistan, where we’re seeing traumatic amputees, burns, multiple high-velocity gunshot wounds. When we go downrange,” — military term for deployment — “everything gets turned upside down.”
Washington University’s partnership with C-STARS ensures new training opportunities in this arena, even as the armed forces wind down their involvement in countries such as Afghanistan and Iraq, Fortuna said.
While St. Louis’ busiest hospital — Barnes-Jewish — is no battlefield, the principles of high-intensity emergency medicine are the same, said Timothy J. Eberlein, MD, the Bixby Professor of Surgery and head of the Department of Surgery. Barnes-Jewish cares for about 13,000 trauma patients each year, with a 99 percent survival rate.

Timothy J. Eberlein, MD, center, head of the Department of Surgery, leads a cohort of military personnel on a tour of the Medical Campus. Above, Maj. Gen. Robert D. McMurry Jr., left, and Brig. Gen. Mark A. Koeniger.
“Not many of us get exposed to high-percussive bombs and armor-piercing bullets like our troops do,” Eberlein said. “But it’s the principles that we all benefit from: How do you stem blood loss? How do you treat the person who has had multiple traumatic injuries? How do you quickly get them out of the field and into a triage of care, in a stepwise progression?”
St. Louis is the oldest C-STARS site, established 15 years ago in partnership with SLU. With Washington University’s entry into the program, C-STARS can leverage the medical school’s many strengths, Eberlein said. Training now takes place on both campuses, and Washington University has plans to recruit more emergency physicians and trauma surgeons in connection with the program.
Eberlein added that the medical school has a long track record of translational research on acute and critical care, trauma surgery and cooperation with the U.S. Defense Department, plus a strong research program focused on traumatic bleeding.
“C-STARS brings concepts from the military that we can adapt, and it makes Washington University and the Department of Surgery better,” said Eberlein, who lobbied for the collaboration. “But there are things we do that are going to help them. It’s an exchange. It really is a partnership.
“These are women and men who put their lives on the line, and develop the casualty programs that take care of our troops. If we can help in keeping their skill levels at the highest level, well, that’s a way for us to give back, frankly.”
How to save a life
As part of the care continuum, the wounded soldier in Afghanistan is moved again — from the austere tent to a Combat Support Hospital at Kandahar Air Base.
After another surgery, he flies on a cargo plane to Landstuhl, Germany, home of the only Level 1 trauma center not on U.S. soil to be certified by the American College of Surgeons. The next day, teams of medics and military workers ready the soldier for his flight across the Atlantic Ocean. Injured U.S. troops ultimately are sent to Walter Reed National Military Medical Center in Bethesda, Maryland; Brook Army Medical Center in San Antonio; or Balboa Naval Medical Center in San Diego.
“With the whole operation running, it would not be uncommon for somebody to be injured on the battlefield and wake up in 72 hours in the U.S., having received three, four or five operations in that time,” said Fortuna, who served two tours of duty in Afghanistan. It’s one reason why, in the most recent conflict in Afghanistan and Iraq, the U.S. armed forces experienced the lowest case fatality rate in the history of modern warfare, he said.
“If you made it off the battlefield, and made it to that tent hospital with a surgeon, your survival rate was over 98 percent. We were actually exceeding civilian hospitals, and with a higher acuity level,” Fortuna said.
It wasn’t always this way. Almost one-fourth of Americans who died in conflict during the past decade perished from wounds they might have survived given the proper care, according to a report from the military’s Joint Theater Trauma System. This finding led the secretary of defense to impose standards for pre-hospital transport times in an effort to reduce death from severe bleeding.
Hemorrhagic shock is defined as a loss of more than 20 percent of the body’s blood supply. At its most basic level, bleeding occurs because blood vessels have ruptured. Injury, surgery, childbirth, infections and cancer therapy all can cause or promote serious bleeding. In the U.S., about 30,000 people die each year from preventable conditions after their initial injury, often due to inadequate therapies or untimely access to care.
If physicians can stop this bleeding, seal torn vessels and replace lost blood, they can save a person’s life. Washington University researchers are searching for novel ways to do this, including improved blood products, artificial blood and blood-clotting mechanisms.
The case for whole blood
During Operation Iraqi Freedom in 2004, critical care doctor Phil Spinella, MD, and his Army colleagues couldn’t wait any longer for blood supplies to arrive in Baghdad. They rolled up their sleeves and gave their own blood directly to the casualties. Surprisingly, it worked better; hemorrhagic shock resolved sooner in patients receiving whole blood versus separated blood components (platelets, red blood cells and plasma) shipped from home.
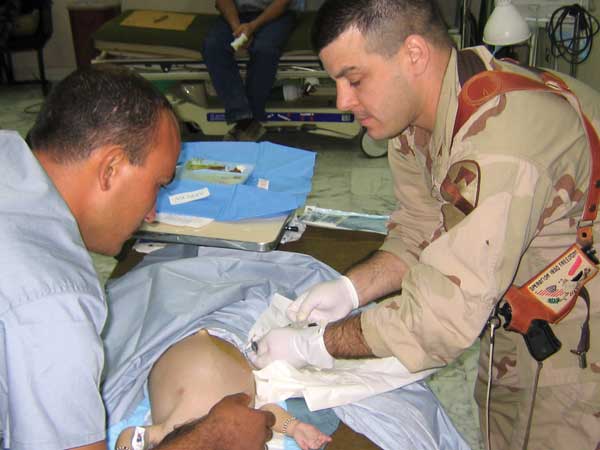
During Operation Iraqi Freedom in Baghdad, critical care doctor Phil Spinella, MD, right, first became interested in the efficacy and safety of blood products for the treatment of shock.
“When you take blood and split it into components, it’s much more diluted. That affects blood’s ability to stop bleeding and to carry oxygen,” said Spinella, who served as active-duty Army for 12 years, earning the Bronze Star and Combat Medic Badge for providing care under fire.
It was during this deployment that Spinella became interested in the efficacy and safety of blood products for the treatment of shock.
Today, he is director of the Pediatric Critical Care Translational Research Program at St. Louis Children’s Hospital, director of the Blood Research Program and professor of pediatrics. He studies transfusion outcomes in children and adults with severe bleeding and the effects of red blood cell storage times on recovery.
Based on research and testimony provided by Spinella, the U.S. surgeon general issued changes supporting increased ratios of both plasma and platelets to red blood cell units for military casualties with severe hemorrhage.
Nationally, Spinella has been a vocal advocate for trauma care improvements. As part of a National Academy of Sciences committee, he helped write a 400-page report to ensure that lessons learned from the military’s experiences in Afghanistan and Iraq are integrated into future combat operations and into the civilian trauma system.
And following the Boston Marathon bombing and resulting concerns about inadequate blood supplies in the wake of terrorist attacks, Spinella organized a full-day conference at the White House for the National Security Service. Spinella said he felt it was important to expose the fact that all major U.S. cities are at risk of running out of blood in any mass-casualty event. Most emergency preparedness efforts focus on evacuations, he said, not on the blood supply. He advocates a whole-blood program in which pre-screened donors could be alerted to report to U.S. collection centers.
Substitutes for real blood
For more than 80 years, researchers have searched for artificial blood substitutes. The need is clear: in acts of mass violence, on the battlefield and in remote locations. Now, the School of Medicine is on the forefront in developing them.

Allan Doctor, MD, left, lead developer of ErythroMer, and research specialist Shivam Shah compare the vascular reactivities of artificial and real red cells. Emulating red cell physiology is a novel, unique feature distinguishing ErythroMer from other blood cell substitutes.
Researchers here believe that red blood cells — in a powdered form — might be available to trauma patients within 10 years. This powder could be stored in a backpack for a year or more and reconstituted with water when needed. By contrast, fresh blood can be stored for about 40 days. Unrefrigerated, it only lasts a few hours.
“It’s not intended to replace human transfusion, but to push transfusion capability into more austere settings.”
— Allan Doctor, MD
Allan Doctor, MD, professor of pediatrics, immediate past director of the Division of Pediatric Critical Care and associate professor of biochemistry and molecular biophysics, worked with Spinella, Gregory M. Lanza, MD, PhD, the Oliver M. Langenberg Distinguished Professor of Science and Practice of Medicine, and Dipanjan Pan, PhD, (now at the University of Illinois at Urbana–Champaign) to create an artificial red blood cell that can pick up oxygen and transport it through the body, as a human red blood cell does.

ErythroMer is a red blood cell substitute formulated from purified hemoglobin. A polymer coating is “immune silent,” so anyone can accept the artificial cells regardless of blood type.
The artificial cells, called ErythroMer, are made from purified hemoglobin, the oxygen-transporting component of red blood cells. The cells are freeze-dried into a powdered form. A polymer coating is “immune silent” so anyone can accept the powdered blood cells regardless of blood type.
Of course, red blood cells perform many functions beyond carrying oxygen. Artificial blood never will replace the real thing, but it can buy critical time for a wounded patient.
“It’s not intended to replace human transfusion, but to push transfusion capability into more austere settings,” Doctor said.
Clinical trials in small animals already have shown success, and the researchers are moving into larger animal studies.
The researchers’ vision extends beyond the battlefield. Such a substitute could be stored where people congregate — in stadiums, malls and other public venues that might fall under large-scale terrorist attacks.
Stopping the bleeding
Washington University researchers also are involved in a variety of blood-clotting innovations.
Spinella and Grant Bochicchio, MD, MPH, the Harry Edison Professor of Surgery and chief of the Section of Acute and Critical Care Surgery, are studying the use of a tranexamic acid, a blood-clotting drug that could improve survival.
The trial, Tranexamic Acid Mechanisms and Pharmacokinetics in Traumatic Injury (TAMPITI), received the first consent exemption ever granted by the School of Medicine’s Institutional Review Board. Patients who arrive at Barnes-Jewish Hospital with traumatic injuries requiring transfusion are automatically placed in the trial, and informed that they received the drug after they are stabilized.
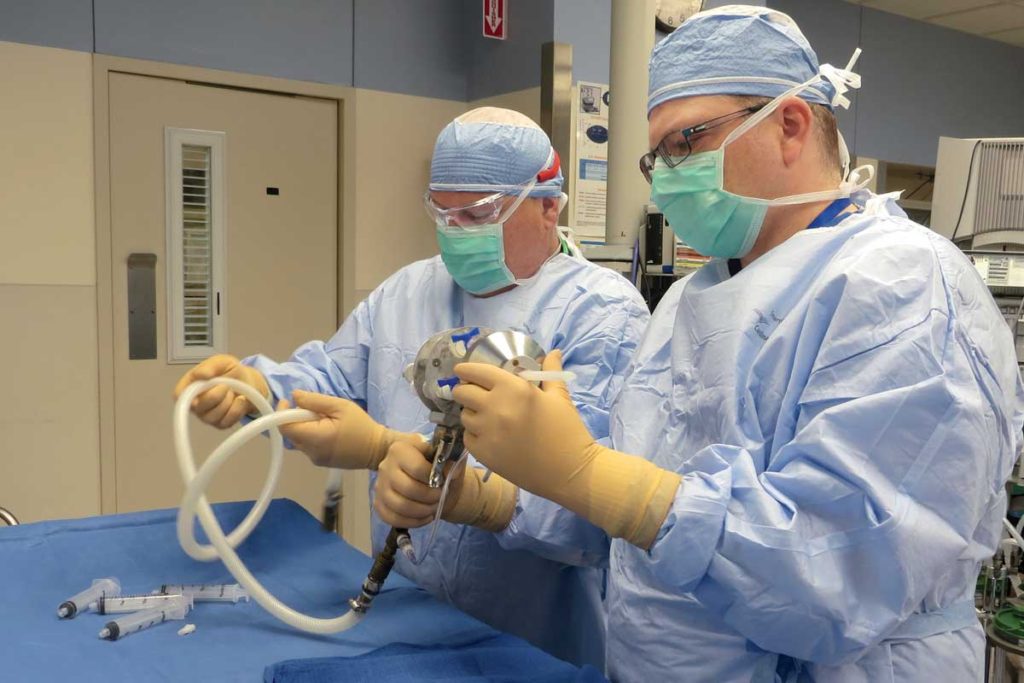
Grant Bochicchio, MD, MPH, and a colleague assemble the device used to apply a specialized foam to control severe bleeding in trauma patients. Bochicchio is heading a clinical trial testing its safety and effectiveness in humans.
Sometimes a drug might not be enough to help clot a wound; in that case, the FDA-approved EVARREST patch could help. It looks like ordinary cotton gauze, but the patch contains blood-clotting agents, so it not only seals a wound, but also actively works to stop blood from flowing, explained Bochicchio, who was involved in the development.
In addition, use of specialized foam to stop severe internal bleeding due to trauma is a step closer to reality, largely through Bochicchio’s efforts.
He is principal investigator of a phase I clinical trial evaluating the safety of ClotFoam. The foam, administered via an applicator device during surgery, binds to the bleeding site and fills the body cavity.
“That way, you can get the patient to a surgeon further afield. It’s kind of like ‘fix-a-flat’ technology, if you will,” he said. Bochicchio also has worked at the R. Adams Cowley Shock Trauma Center in Baltimore, which is another C-STARS center.
Bochicchio and colleagues already have established the foam’s effectiveness in animals; their clinical trial evaluates safety and effectiveness in humans.
In the future, a wounded soldier like the one in Afghanistan might face better odds because of technology and protocols developed or tested in St. Louis — from a transfusion of ErythroMer or whole blood, to the EVARREST patch and ClotFoam, to seamless triage and transport.
While all these changes would improve a wounded war fighter’s chance of survival, the truth is that these advances benefit civilians, too. Spinella said all types of patients could have better outcomes — adults and children alike, trauma and surgical patients and those with internal bleeding.
“This affects everybody, no matter who you are.”
![]()
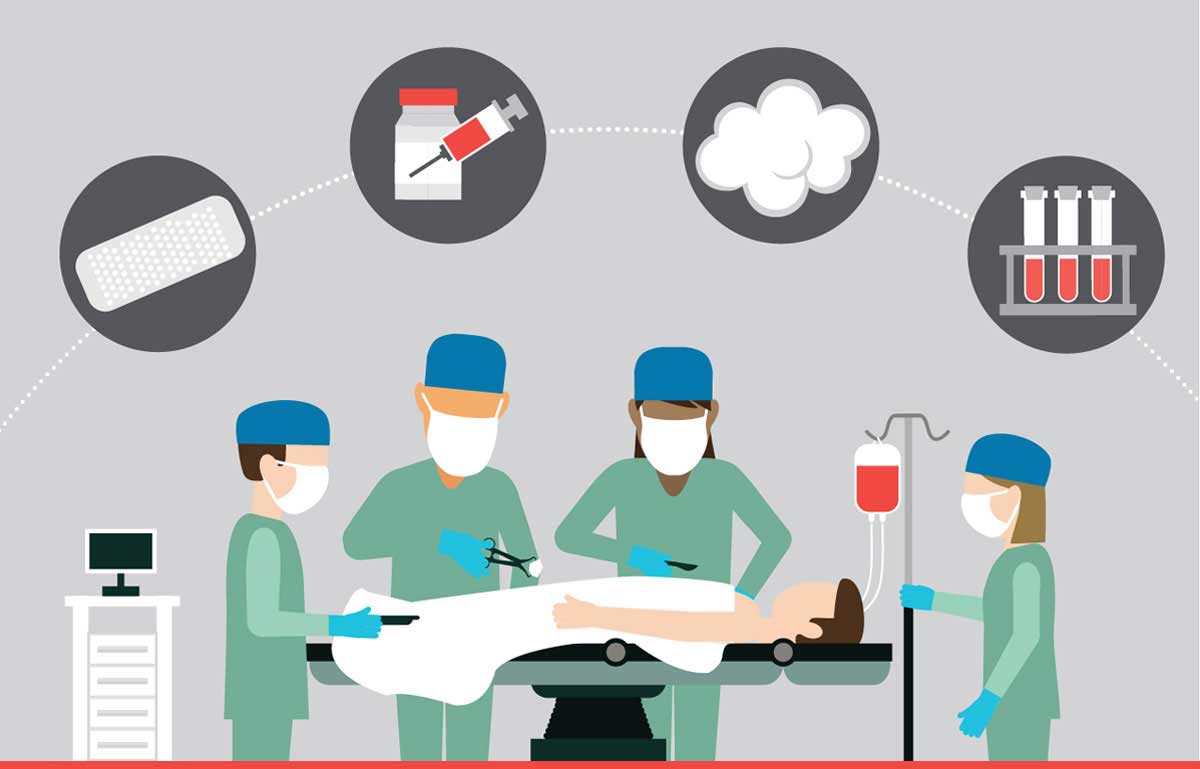
Innovations in blood control and replacement
New options buy time, increase the odds for trauma patients

EVARREST contains two human biological components: fibrinogen and thrombin. When applied to a bleeding area, moisture causes the substances to react together, leading to rapid clot formation. In about eight weeks, the body absorbs the patch material. EVARREST does not require preparation, mixing, moistening with saline or refrigeration, and has a shelf life of 30 months.

Tranexamic acic, or TXA, reduces the breakdown of clots that form with bleeding and has been shown to minimize blood loss in patients with hemophilia, a genetic clotting disorder. Researchers are evaluating the drug’s safety and optimal dosage in trauma patients. Patients who require at least one unit of blood or immediate transfer to an operating room are eligible for the trial.

ClotFoam might help in cases in which a patient is bleeding so badly that compression of the wound doesn’t help, in the operating room and on the battlefield. When mixed and injected, the adhesive hydrogel spreads throughout the body cavity, sealing lacerated tissue and promoting clotting. The product’s technology generates a 3-D scaffold that carries a fibrin sealant.
This nanoparticle-based red blood cell picks up oxygen from the lungs and detects where it should be delivered based on the pH level of the blood. The cells are made from purified human hemoglobin and coated with synthetic polymer. As hemoglobin can cause blood vessel constriction — increasing the risk of heart attack and stroke — the coating is a key differentiator to past attempts.
Published in the Spring 2017 issue





 Share
Share Tweet
Tweet Email
Email