
When you live with dementia, your sleep breaks apart, the nights a strobe-lit blur, the grayed days lost to catnaps. Physicians — and families — have known this for years. But what no one realized, until landmark research at Washington University in 2009 set a series of studies in motion, was that fragmented sleep might be as much a cause as a consequence of dementia. And good sleep in middle age just might ward off a decline.
Sleep disturbances are not normal and inevitable parts of aging, despite what conventional wisdom might say. Often they are early signs of a condition that is treatable.
Dementia is not an inevitable consequence of aging, either. But it is associated with aging — about one-third of Americans over 85 live with some form of dementia. In the U.S., more than 6 million people are living with dementia, and it impacts their ability to think and remember, their personalities and their sense of identity and well-being. The disease also impacts the lives of all who love them. The National Institutes of Health (NIH) predicts this number could double in the next 40 years, as the population grows older and lives longer.
Alzheimer’s disease is the most common cause of dementia. Though symptoms can be temporarily improved, there is no way to prevent the disease or halt its progression.

Except — that landmark study in 2009 opened a channel. Its principal investigator was David M. Holtzman, MD, the Barbara Burton and Reuben M. Morriss III Distinguished Professor, scientific director of the Hope Center for Neurological Disorders, and associate director of the Charles F. and Joanne Knight Alzheimer Disease Research Center (ADRC). Back in 2009, he was not yet thinking about the role sleep might play. He wanted to understand what causes the amyloid beta protein to build up in the brain, contributing to the development of Alzheimer’s.
“There are no walls here. We probably have more people working on the intersection between sleep and neurodegenerative disease than any other research institution.”
David M. Holtzman, MD
Holtzman’s team studied mice, checking their amyloid beta levels as they were awake and moving about or slept, and found that amyloid beta was higher when the animals were awake and active. The longer they were awake, the more amyloid beta they released. When they were given a medication that induced sleep, that suddenly lowered the amyloid beta level. Sleep deprivation increased it again — all that awake time, their tiny brains buzzing.
Normally, amyloid is harmless and floats freely throughout the brain. “It’s probably just a waste product of a larger protein,” Holtzman said. But a buildup of amyloid beta leads to the formation of amyloid plaques — think of them as weighted blankets draped outside the brain cells. They might cause no symptoms for years, but their presence can accelerate a more ominous process: an accumulation of the protein tau, which begins to clump up and tangle inside the neurons, eventually killing the cells and causing that area of the brain to shrink.
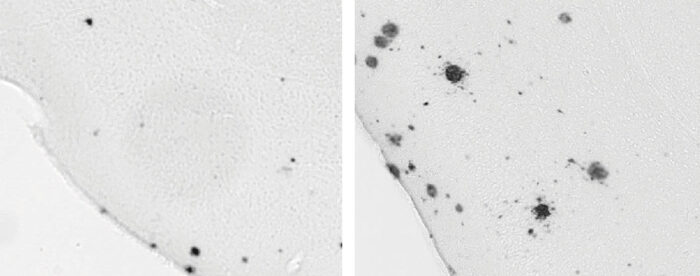
Waking the mice again and again caused both amyloid and tau levels to shoot up to daytime levels due to neurons releasing more amyloid beta and tau during the time when animals were awake and particularly if they were sleep deprived. “Then we wanted to see whether, if you start developing tangles, whether these pathologies found in Alzheimer’s disease cause worse sleep,” Holtzman said. “And we were able to show that amyloid and tau pathologies both worsen sleep.” This was the very definition of a vicious cycle, with sleep deprivation causing brain pathology that then made it even harder to get good sleep.
Holtzman’s amyloid research was published just as Yo-El S. Ju, MD — now the Barbara Burton and Reuben Morriss III Professor — was finishing her residency. “How can we look at this in people?” she asked him. They designed a study with 142 cognitively normal participants from the ADRC, most of whom were part of the Adult Children Study that WashU already was monitoring for Alzheimer’s. Half of the 124 had a parental history of late-onset Alzheimer’s.
Ju’s team monitored the participants’ sleep and wake times using a wearable, watch-like device. The team then compared biomarkers, such as protein levels, found in cerebrospinal fluid. “We found that people who had worse quality sleep were more likely to have other evidence of preclinical Alzheimer’s,” she said. Just like Holtzman’s mice, the individuals with consistently fragmented sleep had amyloid buildup in the brain, even though their thinking and memory were still normal. Poor sleep was itself an early biomarker for Alzheimer’s, showing up years before names, thoughts and memories began to dissolve.
The results were published in 2013. “It was the first paper that showed a link between preclinical Alzheimer’s and sleep in humans,” she said, “and that result has now been replicated multiple times.”
The human volunteers’ circadian rhythms were off, too. That day-and-night pattern is set, not by our Apple watches, but by a master clock in the brain. Circadian rhythms orchestrate a startling array of biological processes, varying, by time of day, the way we absorb sugar, our body temperature, our blood pressure, hormone levels, immune response, and dozens of other physical responses. While not identical with sleep-and-wake cycles, circadian rhythms affect our energy by day and our sleep at night. The study volunteers with high levels of amyloid and tau had fragmented activity patterns, feeling sluggish when the sun shone and restless while the world slept.
Clearly, early Alzheimer’s was linked to quality of sleep. The path for future research had opened.
The right amount of sleep
If sleep is that important, should those of us who toss and turn take sleeping pills? “That is rarely our first recommendation,” said Brendan Lucey, MD, an associate professor of neurology and the director of Washington University’s Sleep Medicine Clinic. For those who want to ward off later dementia by getting more restful sleep, he recommends a regular sleep routine that allows plenty of time for sleep; for patients whom sleep eludes, the first step is to find out why.
But Lucey is studying whether suvorexant, an FDA-approved sleep medication (Belsomra), can help prevent Alzheimer’s disease in a phase 2 clinical trial. In one of Holtzman’s mouse models, a drug in the same class as suvorexant inhibited the formation of amyloid plaques. Lucey is hoping for the same results in humans.
Meanwhile, Lucey began monitoring more than 400 people in 2014, testing whether poor sleep quality is a biomarker for future cognitive impairment. Published in January 2019, the results showed that when people get less restful, deep, slow-wave sleep, tau levels increase.
The data also drew a U-shaped link between amount of sleep and cognitive performance. “There’s a sweet spot,” Lucey explained — roughly six or seven hours of sleep a night — “where cognitive performance was stable.” When people slept less than that — or more — cognitive performance declined over time. The brain knows just how much deep rest it requires.
In another of Lucey’s studies, the research team continuously sampled cerebrospinal fluid (think of doing a spinal tap and leaving the tube in) while doing various sleep interventions. When subjects were sleep-deprived, amyloid and tau levels rose 30% to 50%. “If this is happening night after night for years,” he said, “sleep deprivation could be playing a role in the progression of Alzheimer’s.”
Eventually, he hopes to solve the maddening chicken-or-the-egg puzzle. We still don’t know, he said, “if the sleep disturbance is causing Alzheimer’s, or if it is an early marker that shows that Alzheimer’s is developing. I think it likely could be both.”

Other clues, other conditions
When she is not analyzing sleep’s effect on Alzheimer’s proteins, Ju studies a different protein, this one involved in rapid eye movement (REM) sleep behavior disorder (RBD). RBD is a rare condition in which people act out their dreams as they sleep. Their gestures — unselfconscious, uninhibited, theatrical — are fascinating, but what they imply is grim. “Telling someone they have REM disorder is generally the worst news I have to give,” Ju said, explaining that a high percentage of these patients go on to develop Parkinson’s disease or Lewy body dementia, “and the treatments we have now for RBD will not slow or stop that progression.”
RBD, Parkinson’s, and Lewy body dementia all fall into the category of Alzheimer’s-related disorders, and they are all characterized by the clumping of proteins. With RBD, the crucial protein is alpha synuclein, and again, the problem is overaccumulation. Normally, excess would be cleared away by enzymes within the cells. That’s not happening, so either too much is being produced, or the enzymes are somehow being prevented from clearing it — or both. The clumps, called Lewy bodies, can lead to either Parkinson’s or Lewy body dementia. WashU leads the North American Prodromal Synucleinopathy Consortium for RBD, which follows over 350 individuals with RBD across 10 sites in the U.S. and Canada, to better understand the disease process and develop preventive treatments.
Ju also hunts for clues in common, everyday sleep disorders. Obstructive sleep apnea, for example, interrupts breathing, stuttering the flow of oxygen to the brain, and it also disrupts the restful slow-wave sleep stage, so you wake feeling as though you haven’t slept at all. Slow-wave sleep is the neurons’ chance to rest, letting the brain clear away all the waste products of its mental activity, so apnea disrupts that respite as well. Untreated, it causes inflammation that both increases the risk of dementia and hastens its arrival: People develop cognitive impairment about 10 years earlier, on average, than those without apnea. “If we can identify how apnea is leading to inflammation and how that contributes,” said Ju, “we can target the cause, going beyond treating the apnea to address some of these downstream consequences.”
Research synergy
Dementia has a slippery list of causes and exacerbations, and not everyone agrees that sleep quality plays a pivotal role. A paper published last year in The Lancet did not even include sleep in a list of preventive measures for dementia. But the evidence is building fast.
“Multiple layers of research are happening at WashU, all at the same time,” Lucey said. “That sort of synergy doesn’t exist on this level anywhere else.” Measurements collected in one study form the basis of multiple new studies, and steadily, a scaffolding rises, supporting investigations into new ways to predict and diagnose dementia and new treatments that could halt its progression — or stop it altogether.
To continue moving basic science closer to care, WashU’s new Center on Biological Rhythms and Sleep is pulling together ongoing research in sleep, circadian rhythms, Alzheimer’s and other human diseases. “There are no walls here,” Holtzman said. “We probably have more people working on the intersection between sleep and neurodegenerative disease than any other research institution. And the collaborative spirit is better than I’ve seen anywhere.”
Ten years out, he hopes that “we will be able to affect sleep in some way that will reduce disease risk. And that we can use different sleep assessments to determine whether a treatment is having an effect, instead of measuring only someone’s memory. We want to be able to measure before cognitive decline starts, and then, if we give a treatment, know right away if it is helping.”
Circadian rhythms and Alzheimer’s

Circadian rhythms and sleep cycles are both tied to the rising and setting sun, but the two operate independently. Pull an all-nighter, and your body’s rhythms will not join in; your immune system will still sneak away for a break in the middle of the night, and your gut will slow down, too. Though circadian rhythms are set by a master clock tied to daylight, every human cell — including neurons — has a clock of its own.
“Circadian rhythms are found in all our organs, our blood pressure, our hormones, our gut, our eyes,” said Erik S. Musiek, MD, PhD, the Charlotte & Paul Hagemann Professor of Neurology. He compares all these little 24-hour clocks to musicians in a symphony orchestra: Harmony is only possible when they are all in sync.
In 2011, Musiek joined Holtzman’s lab, one of the few labs studying the role of sleep in Alzheimer’s disease, and he chose a related project: how circadian rhythms affect brain function.
One of Musiek’s early studies yielded a surprise: The gene for the protein YKL-40 was highly regulated by circadian clock genes. He recalled that previous research from the Holtzman lab had linked YKL-40 to Alzheimer’s disease.
Everything connected. Alzheimer’s was linked to disrupted sleep. High YKL-40 levels were linked to Alzheimer’s. YKL-40 was linked to the circadian clock — which, if thrown off, disrupts sleep. Alzheimer’s also is characterized by chronic inflammation — which is controlled by the circadian clock.
Musiek’s team kept going. They found that mice who lack the YKL-40 gene have less amyloid in their brain. Then a WashU geneticist — Carlos Cruchaga, PhD, the Barbara Burton & Reuben M. Morriss III Professor — found that humans with lower expressions of YKL-40 have a slower progression of Alzheimer’s, presumably because there is less amyloid clogging their brains. Why would somebody with high levels of YKL-40 have more amyloid? Because YKL-40 stops the microglia, the brain’s immune cells, from cleaning up any excess.
Disrupted sleep also increases amyloid plaques. Is the disruption caused by a circadian clock that’s lost the beat? Scientists don’t know yet. But they do know the problem isn’t a simple lack of sleep time. When Musiek’s team genetically altered the circadian rhythms of mice, they took so many catnaps, they were getting slightly more sleep than usual, yet their amyloid plaques still increased.
In an idyllic pastoral life, we would naturally rise with the sun and sleep through the darkest night. But in a world filled with glowing screens and bright street lamps, angst and overwork, slumber often eludes us.
What if we learn to keep our circadian rhythms in tune? What if, starting in middle age, we optimize them, improving our sleep quality?
“We think you would have less Alzheimer’s,” Musiek said simply.
Published in the Summer 2022 issue





 Share
Share Tweet
Tweet Email
Email