
Moving innovations out of the so-called ivory tower and into the public domain holds enormous power to treat disease and improve quality of life.
But while academic researchers and physicians may imagine promising clinical solutions, some are unprepared to navigate commercialization: pitching themselves, attracting investors, wrangling with intellectual property law, designing rigorous proof-of-concept studies, locating a commercial space and hiring talent.
Taking an idea from the lab bench to market typically spans a decade.
Washington University is working to empower faculty inventors — paving pathways, clearing obstacles and dedicating significant resources to technology transfer.
At the School of Medicine, entrepreneurial momentum is surging. Leaders say the institution has only begun to realize its potential.

Undeniable impact
In hospitals worldwide, people depend on WashU technology to determine whether a loved one has experienced a heart attack. The gold standard test for heart attack detection originated nearly 35 years ago, when clinical chemist Jack H. Ladenson, PhD, and collaborators invented the first monoclonal antibodies to enable rapid, accurate diagnosis from a blood sample.
Ladenson’s work still forms the basis of such diagnostic tests, affecting many millions of people. It remains the No. 1 commercialization piece to emerge out of the university.
Today, other products developed at the medical school are revolutionizing health care. PrecivityAD, the first blood test for Alzheimer’s disease, received FDA breakthrough device designation this year. Until now, clinical trial participation for many Alzheimer’s studies hinged on time-consuming, expensive brain scans. With the blood test, researchers could screen thousands of potential clinical trial participants per month. “This will help us find treatments faster, and could have an enormous impact on the cost of the disease as well as the human suffering that goes with it,” said product co-developer Randall J. Bateman, MD, the Charles F. and Joanne Knight Distinguished Professor of Neurology.

In the aftermath of stroke, many patients experience hand or arm paralysis. It’s long been assumed motor impairments are permanent at the six-month mark. Eric C. Leuthardt, MD, professor of neurosurgery and chief scientific officer at Neurolutions Inc., is shattering those assumptions. The FDA granted market authorization for Neurolutions’ IpsiHand Upper Extremity Rehabilitation System, a first-of-its-kind brain-computer interface technology licensed from the university. Stroke patients wear a robotic exoskeleton on their hand and wrist, allowing them to regain significant arm and hand function by using their minds. As new neural connections form, the device is no longer needed.
A university imperative
The medical school, a top-five National Institutes of Health (NIH)-funded powerhouse, spends nearly $1 billion annually on research. “Taking our research and getting it into the clinic is critical,” said David H. Perlmutter, MD, executive vice chancellor for medical affairs and the George and Carol Bauer Dean of the School of Medicine. “We believe in the idea that research can improve health outcomes, decrease health-care costs and improve the regional economy. Here, we’re talking about licensing technology, starting and attracting companies and employing people.
“We’ve had incredible success in understanding disease and advancing knowledge. Now we want to lead in commercializing our research.”
Chancellor Andrew D. Martin, PhD, tasked Perlmutter to expand tech transfer initiatives universitywide, as part of his executive vice chancellor role. In August, the university appointed Dedric Carter, PhD, as its first vice chancellor for innovation and chief commercialization officer. “Dedric will have a singular focus of leading our effort to create greater impact through commercialization,” Perlmutter said.
Carter, who joined WashU in 2014, has overseen several operational areas, including food service, parking, summer programs and environmental safety, along with the Office of Technology Management (OTM) and technology transfer functions. Carter’s national reputation in translating research to new ventures extends from his time at MIT and launching the National Science Foundation I-Corps. He also is a key university liaison to the Cortex Innovation Community, a 200-acre business and technology hub that sits along the medical school’s eastern border. WashU and BJC HealthCare have taken leading roles in developing this formerly blighted area into a nationally known innovation district. OTM sits prominently in the district’s @4240 Building.
Carter said he was hired seven years ago with the understanding that WashU was seeking to dramatically grow its commercialization activity. “Seven years ago, we were launching three or four startup companies a year. We have reached a high point of seven, and there’s more room to grow, given the substantial research base that we have at WashU,” Carter said. “We realized we’ve come a long way, but future opportunities are significant.
“Our goal is to help people understand that both knowledge for knowledge’s sake and knowledge that moves to a deeper impact on the human condition are both really important in an academic environment,” he added. “We can do a lot of good by thinking about how to commercialize, protect and move some of these research outcomes from the laboratory to life.”
WashU is making substantial investments around technology transfer, with more to come. In addition to its Cortex development efforts, WashU has expanded the budget, staffing level and expertise of OTM, which ushers innovators through the tech transfer process, and increased gap funding dollars for faculty. While government funding or foundation grants cover early-stage science research, university gap funding often covers the rigorous proof-of-concept experiments or feasibility studies needed to attract financial partners and move to subsequent stages.

In his first 90 days, Carter plans to listen closely as he meets with faculty, staff, students and others involved in commercialization. He’s exploring what the university has done historically in tech transfer, evaluating resources available to innovators and determining what additional support is needed.
WashU is using new methods to move potential assets, especially drug candidates, Perlmutter said. These include forming a board of U.S. pharmaceutical leaders, venture capitalists and entrepreneurs to advise the university on tech transfer strategies and investing significantly in the Center for Drug Discovery, which will expand its role and enter labs to help identify early drug targets.
Additionally, some aspects of commercialization are being outsourced to contract research organizations (CROs), which provide expert guidance in biopharmaceutical and assay development, preclinical and clinical research and clinical trials management. This may be a new arrangement for faculty members, Perlmutter said, but it allows the university to focus on finding therapeutics and moving assets forward without needing to hire specialized labor. CROs, for example, are set up to perform experiments over and over, with exact operating procedures to ensure results can be replicated.
The many efforts are paying off. Fiscal year 2020-21 was a record-breaker for WashU tech transfer, marking the seventh year of continuous growth.
In the midst of the pandemic, faculty researchers rapidly devised tools to address the virus. Among other breakthroughs, Michael Diamond, MD, PhD, the Herbert S. Gasser Professor of Medicine, and David T. Curiel, MD, PhD, professor of radiation oncology, worked to develop a COVID-19 nasal vaccine. India-based Bharat Biotech licensed the technology from the university for further development and, now, the vaccine is in phase 2 trials.
Also, a research team at the Elizabeth H. and James S. McDonnell III Genome Institute and the Department of Genetics developed a saliva test for COVID-19 in collaboration with South San Francisco-based life sciences company Fluidigm. This effort expanded and simplified testing capacity. Richard D. Head, director of the Genome Technology Access Center, and Jeffrey D. Milbrandt, MD, PhD, executive director of the McDonnell Genome Institute, led the team.
WashU innovators this past year submitted 242 invention disclosures (first official recording of an invention to OTM), filed 492 patents and signed 149 revenue-generating license agreements. In these agreements, WashU grants intellectual property rights to a third party, either an established company or startup, for development, and the licensee makes financial payments to WashU. The university in FY20-21 earned $60 million in royalties and licensing agreements.
Revenue is shared with inventors and distributed to schools, departments and central administration to support research, education and tech transfer initiatives. With licensing fees from the heart attack test, Ladenson helped establish two undergraduate scholarships and three endowed professorships at the medical school.
In the last five years, 81% of WashU inventions involved medical school participation; 71% came exclusively from the medical school.
To a certain extent, Perlmutter said, this uptick is a maturing realization of the bipartisan 1980 Bayh-Dole Act, which helped launch academic tech transfer offices. The act gave universities ownership rights to federally funded discoveries, but obligated them to protect and commercialize the discoveries and report progress. A 2018 Return on Investment Initiative deemed the legislation successful in driving academic innovation.
“Science is being questioned right now, and universities have a story to tell about how research impacts everyday life through tangible products,” said Nichole R. Mercier, PhD, assistant vice chancellor and managing director of OTM. “Faculty members also are understanding that the funding landscape outside the university is changing, with government funding agencies’ expectations that research be commercialized.”
Mercier often fields tech-transfer questions from prospective faculty hires. “People want to know they can commercialize their work at WashU. Innovation at WashU is a selling point,” she said.
Carter agreed: “We see more and more junior faculty come in who are very engaged on commercialization topics. Questions about licensing and royalty streams are common comparison points with other institutions as they are perhaps thinking about taking a position.”
Breaking down barriers
While some faculty members are enthusiastic about commercialization, others may have no idea where to begin. OTM has refined its operation to make tech transfer accessible to faculty who are balancing multiple time pressures. “The process can be daunting, and we tell people that it doesn’t have to be — and that we’re willing to spend as much time with you as needed to lower the barriers,” Mercier said.
Nearly 30 OTM staff members work to evaluate promising technologies and guide innovators in licensing intellectual property, filing patents, finding funding and launching startups.
A global network of experts recruited by OTM is available to provide direct feedback on ideas. These are potential investors, serial entrepreneurs, people with deep industry expertise — some are alumni — who understand how to get products on the market, and who have agreed to sit down with innovators and answer questions in real time.
Faculty hear firsthand the rationale behind the decision. “We’re educating in the process,” Mercier said. “I understand you think this idea can do x, y and z. But here are the limitations on x and y, so if you focus on z, I think we have something. Can you get more data on z? And that’s a decision point for a faculty member. It’s about creating this as a partnership.”
Biomedical engineer Matthew R. MacEwan, MD, PhD, has started multiple companies based on his prior work in biomedical engineering. While a graduate student at the medical school, he founded Acera Surgical and developed, in conjunction with WashU neurosurgeons, the first FDA-approved nanofabricated surgical material, Cerafix Dura Substitute. The engineered synthetic material is used to seal and heal defects in the membrane around the brain and spinal cord incurred during routine neurosurgical procedures.
OTM introduced MacEwan to investors and mentors who assisted in crafting the vision for the company and the technology. Through these interactions, MacEwan developed other products, including Restrata Wound Matrix. The regenerative material has helped patients recover from chronic wounds and injuries, including one man who had endured an open leg wound for more than a year following reconstructive surgery for head and neck cancer.
The St. Louis ecosystem
It takes a village to recognize nascent science and get it out into the world. One of the university’s largest Cortex collaborators is BioSTL, a nonprofit organization whose mission is to cultivate bioscience companies. WashU purchased and renovated the former St. Louis Post-Dispatch printing plant at 4340 Duncan Ave. that now houses BioSTL and its office and lab space called BioGenerator Labs. The medical school also provides $1.5 million yearly to support the organization.
This 80,000-square-foot incubator provides affordable space and shared specialty equipment, allowing startups to thrive. “Prior to this, St. Louis had very little in place to help products emerging from research institutions,” said BioGenerator President Eric Gulve. “If you’re thinking about a drug to treat disease, you need access to labs and to people who know how to run a company. The average university professor doesn’t have that skill set.”
BioSTL and BioGenerator Ventures, its investment arm, often provide the crucial first money into a potential startup.
At BioSTL, entrepreneurs also can find mentors, grant-writing support and hiring assistance. “We take PhDs and MDs from WashU and give them a crash course in MBA,” said Maggie Crane, BioSTL communications director.

As research advances, the startup can expand from a single employee to hundreds. WashU startups, such as Arch Oncology and WUGEN, have relocated to larger spaces within the building — keeping their tech and jobs in St. Louis.
The university is particularly interested in attracting businesses to St. Louis. “Our home runs are things like we’ve done with Vir Biotechnology,” Perlmutter said. Longtime medical school faculty member Herbert “Skip” Virgin, MD, PhD, was recruited in 2018 as executive vice president, research and science officer at Vir in San Francisco. Now, the company is basing part of its operation in Cortex. “It is a win for Vir because they have a great pool of talent in the region to draw from and it connects them to WashU,” said J. Gregory Barrett, associate vice chancellor of strategic external projects and outreach on the Medical Campus. “For us, it’s great because it brings a major biotech company to St. Louis and is an example for others who might want to make similar moves.”
WashU is continuing to broaden its net, cultivating national and international industry partners. The goal for most startups is to get bought or, in limited cases, to acquire other companies. This way, faculty can stay involved, turn the business operation over to experts, get discoveries out into the world and pursue other initiatives.
Last year, pharmaceutical giant Eli Lilly and Co. purchased Disarm Therapeutics, a biotechnology startup founded by WashU researchers Jeffrey Milbrandt and Aaron DiAntonio, MD, PhD, to speed the development of treatments for neurodegenerative conditions. Lilly paid $135 million upfront.
“Disarm Therapeutics had reached the point in the drug development process where we either needed to raise much more funding ourselves or work with a pharmaceutical company with the infrastructure in place to take this technology to the next level,” said Milbrandt, the James S. McDonnell Professor and head of the Department of Genetics.
Up until 20 years ago, large pharma companies led U.S. drug research and development. But pharma companies merged, the industry contracted, and universities took over most early-stage drug discovery.
“Sometimes we have preliminary validation of a lead molecule, but it can be too early for a pharma company to come in because there is still too much uncertainty,” Mercier said. “Startups are a very important piece of a university’s ability to get ‘shots on goal,’ to get partnerships that lead to bigger partnerships.”
In another ongoing collaboration, Sun Pharma Advanced Research Company (SPARC), based in Mumbai, India, secures licensing for WashU-identified small-molecule drug candidates and biologics. It funds preclinical research at WashU and validates the work at its facilities, in compliance with regulatory guidelines, and shares intellectual property with the university. This arrangement gives partners a peek at promising assets and, ultimately, moves the research from lab to clinic.
A cultural shift
Perlmutter is encouraging department and division leaders to watch for commercial potential. “One of the problems we have is how do we find out what’s going on in our 700 laboratories? And how do we get to it early and accelerate it?” he said.
To nurture a culture of entrepreneurship, tech transfer activity should be woven into the promotion and tenure process, Perlmutter said. Faculty typically are awarded tenure and promotion based on research grants and publications, not patents, startups or licensing revenue. Commercialization efforts should be appropriately honored, he noted.
Many faculty already are embracing the shift. “Great faculty have been driving success,” he said. Mercier agreed. “Our metrics aren’t slowing down. They didn’t slow down in COVID-19. And now with labs reopening, they are just going gangbusters.
“The fire is burning here at WashU.”

Engaging female entrepreneurs
WashU OTM has been recognized for innovative programs addressing the national disparity of female inventors. The office hosts Equalize, a first-in-class pitch competition for academic women entrepreneurs in the U.S., and sponsors Women in Innovation in Technology, an internal program that teaches how to move from basic research to product commercialization.
In 2013, when the office began developing programs geared toward women, WashU had launched 55 startups — all led by men. Now, females have founded three WashU startups and are equally represented on invention disclosures.

Hong Chen, PhD, associate professor of radiation oncology and of biomedical engineering, is working to launch a startup, called Sonobiopsy, which she pitched at Equalize 2021. Chen’s technology allows genetic diagnosis of brain tumors through a simple blood draw following focused ultrasound sonification. Sonification enhances release of brain tumor biomarkers into the bloodstream. Existing surgical biopsy procedures carry risks and aren’t always possible, depending on location. The promising technology may improve survival outcomes.
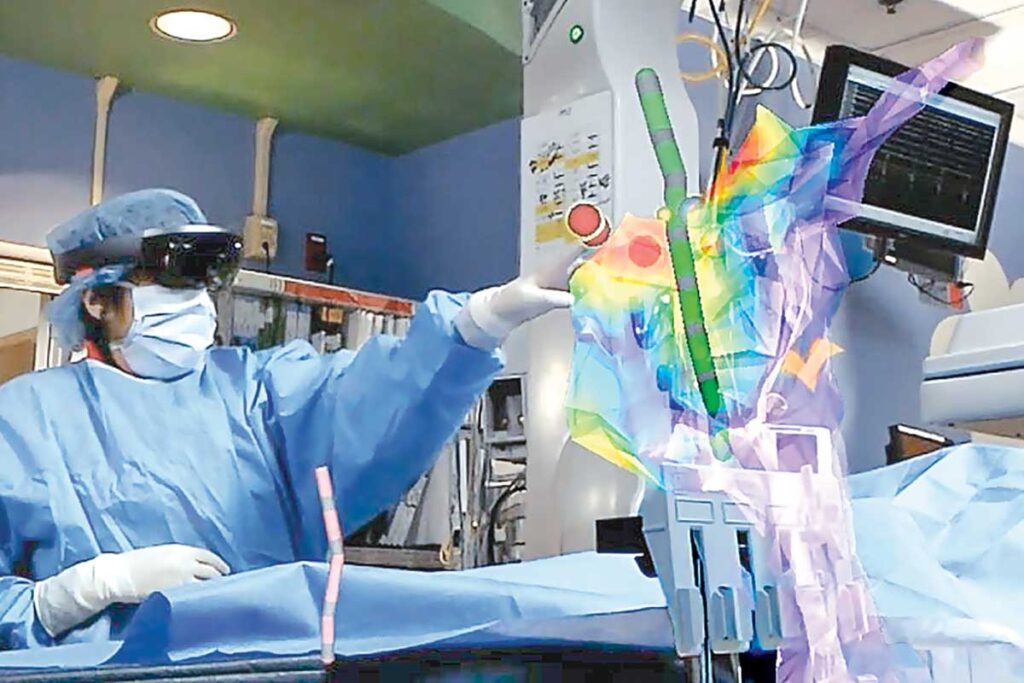
“Perhaps the most important thing OTM has done is to include us in the process and teach us,” said pediatric electrophysiologist Jennifer N. Silva, MD, who invented a 3D augmented-reality platform. “I can touch a million patients this way.”
Published in the Autumn 2021 issue







 Share
Share Tweet
Tweet Email
Email