The brain was once thought to be off-limits to the immune system, too sensitive to tolerate immune cells with their sharp weapons. Now, scientists know that immune cells and molecules flow into and out of the central nervous system all the time, supporting normal brain function, fighting infections and tumors and, yes, sometimes causing injury and disease. The nexus between the immune and nervous systems provides new opportunities to intervene to promote health and prevent or treat neuroinfectious, neuroimmune and neurological conditions ranging from COVID-19 to multiple sclerosis to autism.
Immune surveillance
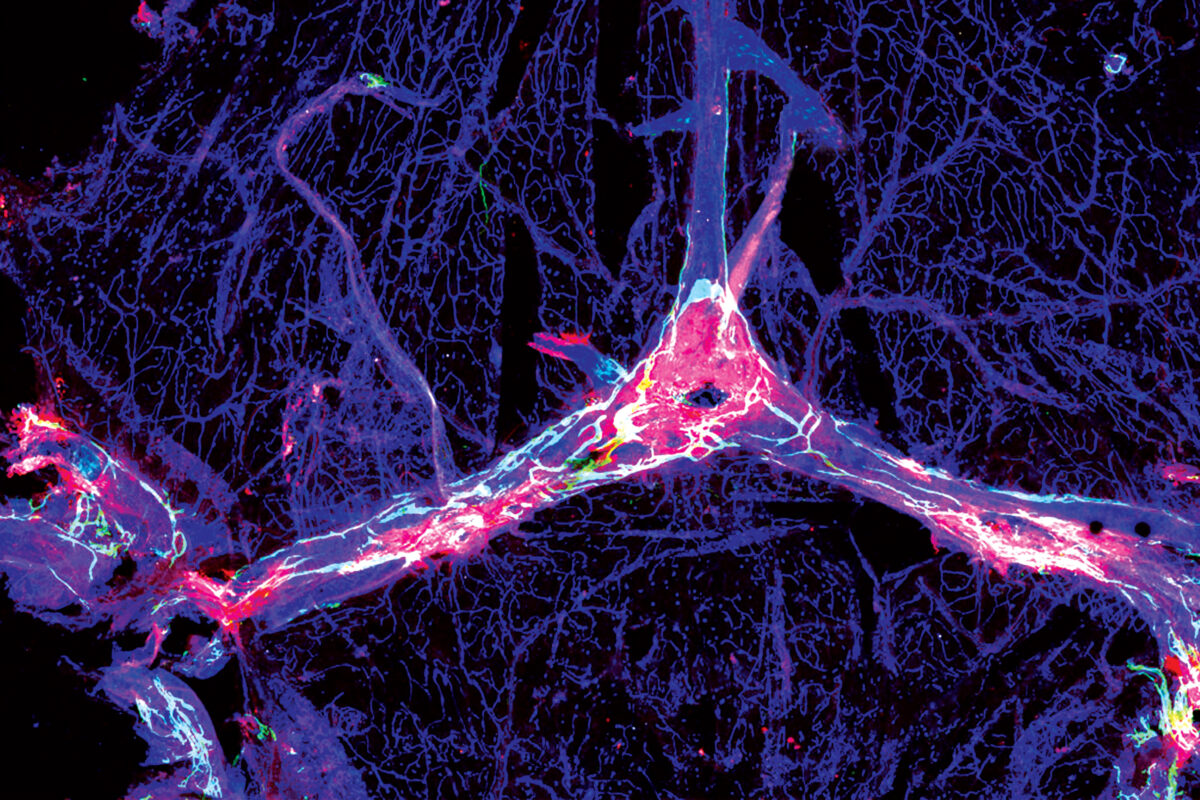
The idea that the immune system surveils and protects the brain is now widely accepted, but until recently it wasn’t clear how and where. In 2015, neuroscientist and immunologist Jonathan “Jony” Kipnis, PhD, found a network of vessels in the meninges — the tissues encasing the brain — that drains fluid and small molecules from the brain into the lymph nodes. Subsequent research indicated that immune cells stationed in the meninges inspect fluid as it washes out of the brain. Such cells are prepared to initiate an immune response if they detect signs of infection or injury, Kipnis said.
“The immune cells that sit on the borders of the brain could potentially be a feasible target for treating neurological diseases such as Alzheimer’s, once we better understand their role in these complex diseases.”
— Jonathan “Jony” Kipnis, PhD
Pandemic brain fog
Before the COVID-19 pandemic made “brain fog” a household term, infectious diseases physician Robyn S. Klein, MD, PhD, grappled with the neurological consequences of another virus: West Nile virus, which can cause memory problems. She found that some West Nile patients’ symptoms worsen even after the virus is cleared because persistent immune activation causes ongoing damage. Long COVID-19, she discovered, was similar. The virus doesn’t infect the brain, but immune molecules released elsewhere spill over into the brain, injuring tissue and causing brain fog. “The same immune response that saved your life could be damaging your brain,” Klein said.
Treating HIV
Before COVID-19 brain fog, there was HAND, or HIV-associated neurocognitive disorder. Since the 1990s, infectious diseases physician David B. Clifford, MD, has led national efforts to understand and treat HAND. Clifford and others have shown that lifesaving antiretroviral therapy reduces but doesn’t eliminate the cognitive consequences of HIV infection, which can include confusion, difficulty concentrating and behavioral changes. “About half of HIV patients in care have some cognitive impairment,” Clifford said. “That is an unreasonable burden that should be reversed as soon as possible.”
Good or bad?
Scientists are just beginning to grasp how complicated the immune system’s role in neurological diseases can be. Take the gene TREM2, which helps control the activity of immune cells in the brain. Immunologist Marco Colonna, MD, and colleagues discovered that, in early stages of Alzheimer’s disease, immune cells lacking TREM2 do a poor job of removing toxic proteins. So TREM2 is good, right? But wait: At later stages, immune cells without TREM2 cause less neurodegeneration, so maybe TREM2 is bad? “Figuring out how to use the immune system to treat neurological diseases like Alzheimer’s is not simple,” Colonna said.
When T cells attack
Multiple sclerosis (MS) occurs when T cells attack the nerves’ insulating sheath, slowing signal transmission and causing fatigue, numbness and miscoordination. But T cells don’t do their damage alone, points out neurologist Anne Cross, MD. Her work has revealed that B cells are essential for turning T cells against nerves, a seminal discovery that led to an FDA-approved MS drug that works by suppressing B cells. Meanwhile, molecular biologist Naresha Saligrama, PhD, is investigating how the T cell populations of healthy people and people with MS differ. “Everyone has some self-reactive T cells, but not everyone has an autoimmune disease. What’s different about people who get MS?” he asked.
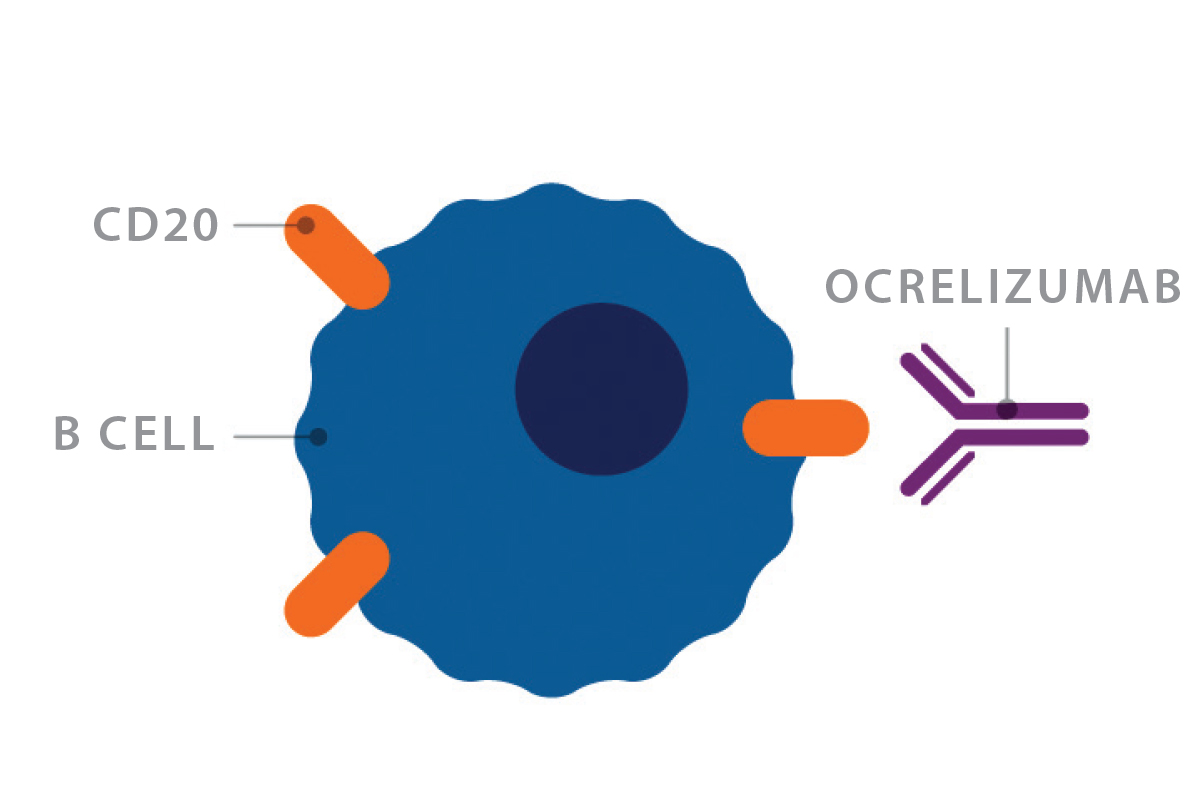
Targeting B cells in MS
While rituximab was never approved to treat MS, it paved the way for a new class of MS therapeutics. The FDA has approved three antibody-based drugs that deplete B cells by targeting the CD20 molecule, a marker exclusive to B cells. Once targeted, the B cell is marked for destruction. Ocrelizumab (right) was the first such drug approved.
Navigate the neurosciences
Continue exploring the extensive scope of neuroscience research conducted at WashU Medicine.
Published in the Winter 2023-24 issue










 Share
Share Tweet
Tweet Email
Email